
Phil
Cleverley
SCOR Chief Underwriter

December 27, 2024
Over the last year, there has been a constant stream of media articles relating to the development of weight loss drugs, with research claiming that significant improvements in weight can be achieved, and an ever-growing list of new drugs being developed and approved for use.
These drugs are known as glucagon-like peptide-1 (GLP-1) receptor agonists medications and are a class of drugs that have for some time been available for managing type 2 diabetes (T2DM) and more recently have emerged as an effective treatment option for weight loss.
As underwriters, we are already starting to see applications with disclosures of where this range of drugs is being prescribed for weight loss and there is no doubt, they will become increasingly seen on medical evidence at underwriting and claims in the future.
Both the UK and Ireland have very high levels of obesity and overweight that for many, are resulting in reduced life expectancy and poor quality of life. Despite the plethora of weight management strategies available, many individuals continue to struggle to achieve substantial and sustained weight loss.
The implications are that this new range of GLP-1 drugs could play a significant role in improving our nation’s health and could impact very positively to improving mortality and morbidity in the UK and Ireland.
The most widely used measure of obesity is the Body Mass Index (BMI), defined as weight divided by the square of height (kg/m²). A person is classified as obese if their BMI is 30 or higher, and overweight if their BMI is between 25 and 30. A BMI of 40 or more is often known as ‘morbid obesity’. Excess weight is an umbrella term for BMI over 25, i.e., either overweight or obese.
Obesity costs the UK National Health Service (NHS) around £6.5 billion a year and is the second biggest preventable cause of cancer based on evidence compiled by Cancer Research UK(1).
An important study from 2020(2) demonstrated that obesity also plays a significant role in hypertension, increased risk from stroke and diabetes and estimated that 65-78% of cases of high blood pressure are caused by obesity.
An NHS public health survey in 2014(3) indicated 90% of adults with T2DM in the UK and 60% of type 1 diabetics were overweight or obese. Data from the 2021 Health Survey for England(4) showed 25.9% of adults in England were obese and a further 37.9% were overweight, making a total of 63.8% who were either overweight or obese. Data for Scotland, Wales and Northern Ireland show similar patterns.
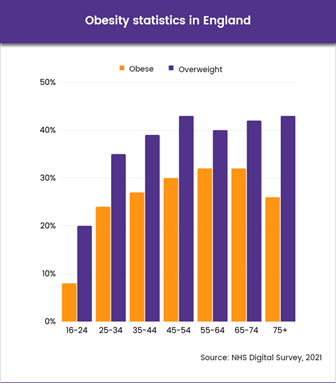
Ireland shows equally worrying statistics, with World Health Organisation (WHO) reporting that a quarter of adults in Ireland are obese, with a total of 61% of adults being overweight or obese. Among five- to nine-year-olds, 34% are overweight or obese. The burden of adult obesity in financial terms has been estimated as €1.13 billion per annum.
GLP-1 medications are a class of medications that mimic the effects of a hormone called glucagon-like peptide-1 that is produced naturally in the body and helps to regulate blood sugar levels by encouraging the body to produce insulin at mealtimes and increasing feelings of fullness. By reducing appetite and increasing fullness, GLP-1 medications can also help people consume fewer calories and promote weight loss.
Currently, there are a number of GLP-1 medications approved for use in the UK, including the following:
These medications are available in both injectable (daily or weekly) and tablet forms, with the injectable forms generally being more effective for weight loss.
There are currently 4 GLP-1 medications in Ireland available for T2DM. Namely, dulaglutide, exenatide, liraglutide and semaglutide. However, Saxenda is the only GLP-1 medication available for weight loss.
GLP-1 medications have been shown to be highly effective in managing T2DM and additionally have been shown to reduce the risk of heart attack, stroke, and death from cardiovascular disease. In clinical trials, GLP-1 medications have been shown to lower HbA1c levels (a measure of average blood glucose levels over time) by up to 1.5%.
Compared to other diabetes medications, GLP-1 medications have several advantages. In addition to promoting weight loss, that is a major risk factor for T2DM, they do not cause hypoglycaemia (low blood glucose), which can be a serious side effect of other diabetes medications. Clinical trials have shown that GLP-1 medications can be highly effective for promoting weight loss. In a study published in the New England Journal of Medicine in 2021(5), researchers found that patients treated with semaglutide, a once-weekly GLP-1 medication, lost an average of 15% of their initial body weight over 68 weeks of treatment. Other GLP-1 medications have also been shown to show similar results.
The latest drug (at the time of writing) “Mounjaro” was originally approved by the National Institute for Care Excellence (NICE) for T2DM in October 2023 and approved by the Medicines and Healthcare Products Regulatory Agency on 8th November 2023 for weight loss. Clinical trials claim it is even more successful than Wegovy in achieving weight reduction in obese or overweight adults (with no T2DM) over a 72-week period of between 16% to 22.5% depending upon dosage.
Mounjaro (tirzepatide) differs from the other weight loss treatments as in addition to GLP-1 it also activates a similar hormone called glucose-dependent insulinotropic polypeptide (GIP) and is the first dual GIP/GLP-1 receptor co-agonist approved for the treatment of type 2 diabetes and weight loss.
Another drug with early exciting results is retatrutide that is considered to be a next-generation weight loss treatment and is a tri-agonist, meaning that as it interacts with three separate hormone receptors – GLP-1, GIP and a glucagon receptor. Currently, retatrutide is in Phase III testing. Results from Phase II clinical trials showed over a 48-week period an incredible weight loss of up to 24% depending upon dosage.
At these levels of weight loss, this drug could potentially impact very significantly in reducing the need for radical bariatric surgeries such as gastric banding or gastric bypass, as well as providing a considerable improvement in quality of life and life expectancy.
As with any medication, there are potential side effects with GLP -1 medications, with common side effects including nausea, vomiting, diarrhoea, and constipation. These side effects often subside over time, as the body adjusts to the medication. Therefore, complications of the treatments are usually not significant. In rare cases, more serious side effects like pancreatitis and thyroid tumours have been reported, though the risks appear to be low.
The drug metformin (Glucophage) is the first line treatment for T2DM and is proven to be very effective as a singular treatment for controlling the condition. However, if control of the condition is maintaining adequate control or there are other complications such as intolerance or contraindications, then GLP-1 medications may be added to the treatment plan. They may also be added for those with T2DM who have other cardiovascular risks including being obese or overweight.
Semaglutide under the brand name of Wegovy was approved specifically as an option for weight loss treatment in the UK on 4 September 2023 and became available on the NHS in line with strict guidance from the National Institute of Clinical Excellence (NICE) to be provided alongside a reduced-calorie diet and increased physical activity.

Wegovy became the first drug approved to target obesity in the UK, limited to adults with at least one weight related condition, such as obstructive sleep apnoea, heart disease or hypertension and a BMI of 35. It may also be used for those with a BMI of 30 to 34 in exceptional circumstances who have a co-morbidity and are eligible for a referral to a specialist weight management service (usually within a hospital).
The approval of Wegovy as a weight loss drug followed the announcement in June 2023 from the UK Government of an investment of £40 million for a pilot scheme that would make the drug available to around 35,000 eligible people. The situation in Ireland is that Wegovy was approved for use as a weight loss treatment by the European Medicines Agency in January 2022. However, it is not currently available with the huge demand that semaglutide has created across the world with the Danish manufacturer, Novo Nordisk, working to make it available soon.
Although Ozempic is a drug primarily manufactured for the treatment of T2DM, it can also be prescribed “off label” for those seeking to lose weight, meaning that because the drug is licensed for use, it can also be prescribed for weight loss because of the known benefits it can provide.
With the increased popularity of GLP-1 medications being used for weight loss, it has resulted in a shortage of Ozempic for those suffering from T2DM in both the UK and Ireland which is anticipated to last well into 2024. In the UK this has led to the Department of Health and Social Care (DHSC) together with the NHS banning prescription of Ozempic for weight loss until supplies improve. The Department of Health in Ireland back in May 2023 produced similar restrictions.
With the shortages of drugs including Ozempic and Wegovy, there are increasing reports of these drugs being sold illegally via the internet as well as being made available in beauty salons with Ireland reporting a 5-fold increase in illegal imports of semaglutide this year when compared to the whole of 2022.
Whilst the benefit of GLP-1 medications is likely to have a positive impact for many, they could equally cause significant issues when these drugs are being taken with no medical supervision. Even more worrying, is the development of counterfeit versions of the drugs being discovered across the globe.
GLP-1 medications prescribed under the guidance of a medical professional and forming part of a weight loss management program with diet and exercise, do not present any additional risks for the underwriter based on the treatment. While there are some side effects associated, these are relatively typical and the risk for serious side effects is low.
If there is a history of rapid weight loss, then the fact that GLP-1 medications may have played a role, should not change any typical underwriting practices that might make some adjustment to current weight allowing for the risk of weight gain in the future.
Underwriters should also be aware of the guidelines for use of GLP-1 medications for both T2DM and obesity/overweight, since there will often be other co-morbidities present that will need consideration.
If it is discovered that GLP-1 medications have been taken outside of supervision from a medical professional, then it should be viewed very seriously in view of the significant risks that can result.
GLP-1 medications such as Wegovy and Mounjaro for weight loss are a very exciting developments that could impact very significantly in reducing weight related mortality in the UK and Ireland.
The research indicates that significant weight loss can be achieved through GLP-1 medications, and it is likely more drugs will be developed that will have even greater potential.
The current global shortage of GLP-1 medications shows just how quickly these drugs have been identified in treating people with obesity and it is still too early to say whether once the treatments end, that individuals will simply put the weight back on again. However, there is no doubt GIP-1 medications will be increasingly encountered by underwriters for the near future.